Gastrulation is the process of cell movements that give rise to the primary germ layers of the embryo. The initial positions and neighbors of the blastomeres is determined by the pattern of cleavages. New positions and neighbor relationships are determined by the pattern of cell movements at gastrulation. Cells can migrate as individuals, eg., ingression, or as part of a unit with other cells, eg., invagination, involution, delamination, and epiboly.

SEA URCHIN GASTRULATION
A fate map can be drawn on the egg because of the stereotyped pattern of cleavages. The same blastomeres end up with the same egg cytoplasm and in the same positions in the blastuala.

Remember the cleavage pattern of sea urchins. The egg is isolecithal and cleavage symmetry is radial holoblastic. Even so, blastomeres do not always cleave symmetrically. Remember at the 4th cleavage the animal pole mesomeres divide in the meridinal plane while the vegetal pole cells divide in the equatorial plane asymmetrically to give rise to the 4 macromeres and the 4 micromeres.
The fate of blastomeres in the hatched blastula can be easily recognized as arising from the early pattern of cleavages and suggests little cell movement during the blastula stage.

INGRESSION OF PRIMARY MESENCHYME
Excellent movie of sea urchin gastrulation from Rachel Fink's "A Dozen Eggs". Movie 1 "primary mesenchyme ingression". About 24 hrs after hatching of the ciliated blastula the vegetal side of the blastula begins to flatten to form the VEGETAL PLATE. The blastula is surrounded by the hyaline layer and the blastomeres have secreted a basal lamina surrounding the blastocoel. The blastomeres adhere strongly to the hyaline layer and to each other. They are also attached at their basal surfaces to the basal lamina.

Centrally located cells begin to extend filopodial processes from their inner surface. The cells loose their adhesion with the surrounding cells of the blastoderm and are pulled inward into the blastocoel by their filopodial processes. The cells INGRESS as individuals and not as a sheet (not epiboly), not an INVAGINATION, not an INVOLUTION, not a DELAMINATION. Cells which are the first to INGRESS are called the PRIMARY MESENCHYME and are derived from the MICROMERES. They are fated to give rise to the SKELETAL RODS of the PLUTEUS LAVA. These cells first migrate randomly in the blastocoel, but soon become localized on the ventral surface of the blastocoel, where they fuse to form the syncytial cables that will give rise to the calcium carbonate spicules.
This model that suggests differing adhesive interactions is responsible for the ingression of the primary mesenchyme was directly tested by Fink and McClay. They first made test plates to which they bound hyaline, test cell monolayers, or basal lamina. They then added 16 cell stage micromeres, migratory stage primary mesenchyme cells, gastrula ectoderm and endoderm. Results showed that pre-migratory micromeres adhered 100 times stronger to the hyaline layer and other blastomeres than to the basal lamina. The migratory primary mesenchyme cells showed just the opposite behavior. They adhered 100 times more strongly to the basal lamina than to the hyaline layer or other blastomeres. An important component of the basal lamina is fibronectin. The affinity of the primary mesenchyme cells for fibronectin increases dramatically at the time of ingression. The primary mesenchyme cells synthesize some as yet unidentified surface sulfated glycoproteins. Both these components, fibronectin and the sulfated glycoproteins, are important for the ingression and migratory behavior of the primary mesenchyme.
FIRST STAGE OF ARCHENTERON INVAGINATION
After a period of migratory exploration the primary mesenchyme cells settle into a ring in the vegetal region of the blastocoel. The cells of the vegetal plate maintain their adhesive interactions with one another and with the hyaline layer. After ingression of the primary mesenchyme the vegetal plate invaginates about 1/3 the way into the blastocoel. The invaginated region of the vegetal plate is called the ARCHENTERON (or primative gut). The indention of the blastocoel at the vegetal pole is called the BLASTOPORE. This first stage of archenteron invagination is due entirely to the intrinsic behavior of vegetal plate cells. Even if the vegetal plate is cut away from the rest of the blastocyst it still undergoes the bending associated with invagination. One possible mechanism is a change in cell shape. The vegetal plate cells expand at their inner (basal) surface and contract at their outer (apical) surface causing the epithelium to buckle inward. Another possibility that is supported by experimental evidence is shown below. Chondroitin sulfate proteoglycan is secreted into the hyalin layer and causes that layer to swell and buckle inward (see diagram)

SECOND STAGE OF ARCHENTERON INVAGINATION
A dramatic 2-3 fold lengthening of the wide short archenteron occurs during the second stage of invagination. There is no increase in cell number, rather there is a rearrangement and change in the shape of the cells. The cells flatten, extend, and migrate over one another to form the thinner and narrower tube of the archenteron.

The secondary mesenchyme cells ingress from the tip of the archenteron. They extend filopodia to the wall of the blastocoel and both pull the archenteron towards the animal hemisphere blastocoel wall and also determine where the archenteron contacts the animal hemisphere blastocoel wall and thus where the mouth will form. The secondary mesenchyme leave the top of the archenteron and disperse within the blastocoel to later give rise to the mesodermal organs.

The contact between the archenteron and the blastocoel wall of the animal hemisphere determines where the future stomadeum (mouth) will form. The fusion of the mouth with the archenteron creates the continous tube of the digestive tract. The blastopore marks the future proctadeum (future anus). Thus the characteristic feature of deuterostomes---blastopore, which forms first gives rise to the anus, while the stomadeum forms second.
Movie 2 "Archenteron formation" from Rachel Fink's "A Dozen Eggs".
GASTRULATION IN FROGS
Remember the pattern of early cleavages in frog. The sperm entry point in the animal hemisphere not only activates the egg, but also determines the orientation of the first cleavage. Sperm entry initiates a cortical rotation of the egg cytoplasm that reveals the grey crescent. The grey crescent is always bisected by the first meridinal cleavage and determines the site of the blastopore (the DV axis).

By the end of the cleavage stage there are many more smaller animal hemisphere blastomeres than the fewer larger yolk rich vegetal pole blastomeres. A blastocoel has developed, displaced towards the animal pole. Notice, that in contrast to the sea urchin, the frog blastula is a multilayered hollow ball of blastomeres. A fate map can be drawn of the late stage blastula, just before the beginning of gastrulation. However, an exterior and interior fate map is needed to illustrate the differing fates of the cells. Notice that it is especially at the equatorial region that the interior cells are destined for mesodermal fates. The blastopore begins forming at the junction between endoderm and mesoderm (notochord). Cells acquiring grey crescent cytoplasm define the DV axis and the site of blastopore formation.





Notice in the figure below how surface ectodermal cells move by epiboly to enclose all the immobile vegetal cells (yolk plug) as the blastopore slowly constricts at the end of gastrulation.

We can do transplantation, isolation, and ablation studies, just as in the sea urchin, to test when cells become determined and how great are the regulative abilities of the embryo at each stage of development. The first experiment in A shows the transplantation of "normal back cells" from a late blastula donor to the position in late blastula host that normally gives rise to belly tissue. Notice that the transplanted tissue takes on the normal fate of cells in the host position demonstating that they had not yet been irreversibly determined as "back cells". In the second experiment, B, a glass needle is used to remove cells from a late blastula (an ablation experiment). The resulting embryo is normal, demonstrating the regulative ability of the late blastula frog embryo.

A similar experiment is shown in A below. Transplantation of presumptive neural plate from a donor early gastrula to the position of presumptive epidermis in the host results in the formation of epidermis. The presumptive (based on fate map) neural ectoderm had not yet been irreversibly determined and was able to respond to local signals from host cells and differentiate normally as epidermis in the host. However, something happens by the late gastrula stage. Now the same experiment demonstrates that the donor tissue had already been determined as neural tissue and now differentiates as ectopic (misplaced) neural tissue in the host position where epidermis should differentiate.

Cells of early gastrula are uncommitted with respect to their eventual differentiation. By late gastrula fates of cells are fixed. As shown by the above experiments, early transplants result in cell fate detemined by host position while late transplants result in fate determined by donor position. Thus between early and late gastrula cell fate is DETERMINED. There is one famous exception! The dorsal lip region of the late blastula/early gastrula has unique properties. The transplantion of this tissue by Spemann and Mangold let to a dramatically different result. The transplanted dorsal lip tissue induced the surrounding host tissue to form a new embryonic axis.

SPEMANN AND MANGOLD and Primary Embryonic Induction.
The dorsal lip of the blastopore is the only self differentiating region in the early gastrula because when transplanted it can initiate gastrulation and affect the surrounding host tissue. Heteroplastic (interspecies) transplantation with two species of newt, one darkly pigmented and the other non pigmented allowed Spemann and Mangold to separately follow host and donor cells derived cells during development. .Transplantation of the dorsal lip of the blastopore at EARLY gastrula stages into novel locations in another early gastrula resulted in the formation of new blastopore, nearby host tissues were induced to gastrulate, and form a new body axis.

Thus, the normal fate of cells could be completely changed by association with cells of the dorsal lip of the blastopore.The result was often a double embryo connected at the belly. This is very different from the other transplants that showed cells were not determined until the late gastrula. This is a dramatic example of embryonic induction.The dorsal lip of the blastopore is call the ORGANIZER. Later in the course we will examine the molecular basis of organizer determination and function.

GASTRULATION IN BIRDS
Remember that cleavage in birds (telolecithal eggs, meroblastic discoidal cleavage) gives rise to the blastodisc sitting atop the massive yolk.



Central cells of the avian blastodisc are separated from the yolk by a subgerminal space and appear to be clear. This region of the blastodisc is called the AREA PELLUCIDA---these cells give rise to the embryo. Cells at the margin appear opaque because of their close contact with the underlying yolk---this region is called the AREA OPACA----these cells are involved in processing the yolk and do not contribute to the embryo proper.
Cells ingress or delaminate from the outer epiblast layer into the subgerminal cavity to form the layer called the PRIMARY HYPOBLAST. Cells from the posterior margin migrate anteriorly to join the primary hypoblast cells. These are called the secondary hypoblast cells


Epiblast and hypoblast are joined together at the margins of the area opaca. The space between them is the now termed the blastocoel. The hypoblast does not contribute to the developing embryo. It forms membranes which line the yolk sac.
The fate map of the avian epiblast compared to zebrafish, frog, and mouse. Notice that topologically they all look very similar.

A characteristic feature of avian and mammaliam gastrulation is the PRIMITIVE STREAK. Cells from the lateral region of the posterior epiblast migrate towards the center. This is visible as a thickening of the epiblast at the posterior central region of the area pellucida.

The underlying hypoplast at the posterior midline (Koller's sickle) induces the formation of the primitive streak. Evidence for this came from transplants of the hypoplast. When the hypoblast was rotated the overlying primitive streak was altered such that the axis was aligned with the hypoblast layer. When cells of the posterior margin that give rise to the secondary hypoblast were removed no primitive streak formed. The cells forming the primitive streak migrate anteriorly causing the primitive streak to lengthen and narrow. The primitive streak extends about 75% the length of the area pellucida and defines the anterior-posterior axis of the animal.



A depression forms within the primitive streak called the PRIMITIVE GROOVE. The primitive groove is analogous to the blastopore. At the anterior end of the primitive streak is a regional thickening of cells called the PRIMITIVE KNOT (HENSEN’S NODE). Its the analogous to the dorsal lip of the amphibian blastopore. The blastoderm cells migrate over the lips of the primitive streak and into the blastocoel. The primitive streak has a continually changing cell population just as the edges of the amphibian blastopore. Ingressing cells are coated with HYALURONIC ACID, (a linear polymer of glucuronic acid and N-acetylglucosamine) which is synthesized by the epiblast cells and secreted into the blastocoel. This coating seems to be essential for the changed adhesive behavior of the ingressing cells. These cells do not adhere to one another, but instead adhere to extracellular matrix molecules within the blastocoel. In contrast to the amphibian gastrulation where sheets of cells involute during gastrulation, avian grastrulation is an INGRESSION of epiblast cell which form a loose MESENCHYME within the blastocoel. There is not a true archenteron as seen in sea urchin and frog gastrulation.
Fibronectin also seems to be important for the migratory behavior of the ingressing cells just as in the sea urchin blastocoel. Fibronectin is secreted by the epiblast cells into the basel lamina coating the upper surface of the blastocoel. The first cells to migrate through the primitive streak are those destined to become the foregut endoderm, just as in the sea urchin and the frog. Inside the blastocoel these cells migrate anteriorly and eventually displace the hypoblast cells in the anterior portion of the embryo. As ingression continues the primitive streak elongates anteriorly. This headward elongation is coincident with the anterior migration of the seconday hypoblast cells from the posterior margin of the blastodisc. The next cells entering the blastocoel through Hensens Node also move anteriorly, but they do not move as far ventrally as the presumptive endodermal cells. These cells remain between the endoderm and the epiblast to form the head mesoderm and the chordamesoderm (notochord) cells. The early ingressing cells thus all move anterior to form the head process.
Those cells passing through the lateral portions of the primitive streak give rise to the majority of endodermal and mesodermal tissues. Ingressing cells along the primitive streak split into two streams. One stream moves deeper along the midline and forces the hypoblast cells laterally. These deep ingressing cells give rise to all the endodermal structures of the embryo in addition to most of the extraembryonic membranes. (hypoblast also contributes to extraembryonic membranes). A second stream of ingressing cells migrates into the blastocoel between the epiblast and hypoblast. These cells form a loose sheet of mesenchyme which will give rise to the mesodermal structures of the embryo and the extraembryonic membranes.

Concurrent with the lateral plate and axial mesoderm ingression the primitive streak starts to regress. As Hensen’s Node moves posterior it leaves the head process and the notochord in its wake. This anterior to posterior movement of gastrulation is correlated with further development along the anterior posterior axis. Head structures are beginning to form while gastrulation is continuing in the more posterior regions. See e and f in figure 11.10 above.

Thus there is an anterior to posterior gradient in development. Hensen’s node regresses to the position of the proctadeum, the epiblast is composed entirely of ectoderm precursors, and gastrulation ends. While ingression of presumptive endodermal and mesodermal cells was occuring the ectodermal precursors were surrounding the yolk by epiboly. The cells of the area opaca are joined to each other by tight junctions and travel as a unit rather than individual cells. The marginal zone cells of the area opaca adhere along their upper surfaces to the the lower surface of the vitelline envelope and spread along this surface to surround the yolk. Proximal cells of the area opaca do not adhere tightly to the vitelline layer.

Transplantation experiments similar to those described by Spemann and Mangold were performed on bird embryos. Tranplantation of Hensens Node (dorsal lip of blastopore) to a new location in a host induces the formation of a new embryonic axis. Just as in the frog, the dorsal lip of the blastopore is the first tissue to be determined and has inductive properties essential for axial patterning of the bird embryo.
GASTRULATION IN MAMMALS

Gastrulation in mammals is surprisingly similar to avian gastrulation even though mammals do not have to deal with the large yolk. Instead of yolk to nourish the developing embryo mammals have evolved a way of keeping the developing embryo within the body of the parent and deriving constant nourishment from the parent. The fetal organ involved in absortion of maternal nutrients and exchange of wastes is the placenta. The placenta is derived primarily from the embryonic trophoblast cells (however there is some contribution from mesodermal (blood vessels) cells derived from the inner cell mass).
ORIGINS OF TISSUES

The first segregation of cells within the inner cell mass involves the formation of the hypoblast layer. These cells separate from the inner cell mass to line the blastocoel cavity where they give rise to the yolk sac endoderm. These cells do not contribute to the embryo.

The epiblast delaminates into the embryonic epiblast and the amnionic epiblast which form the lining of the amnionic cavity. Embryonic epiblast contains all the cells that generate the embyro.
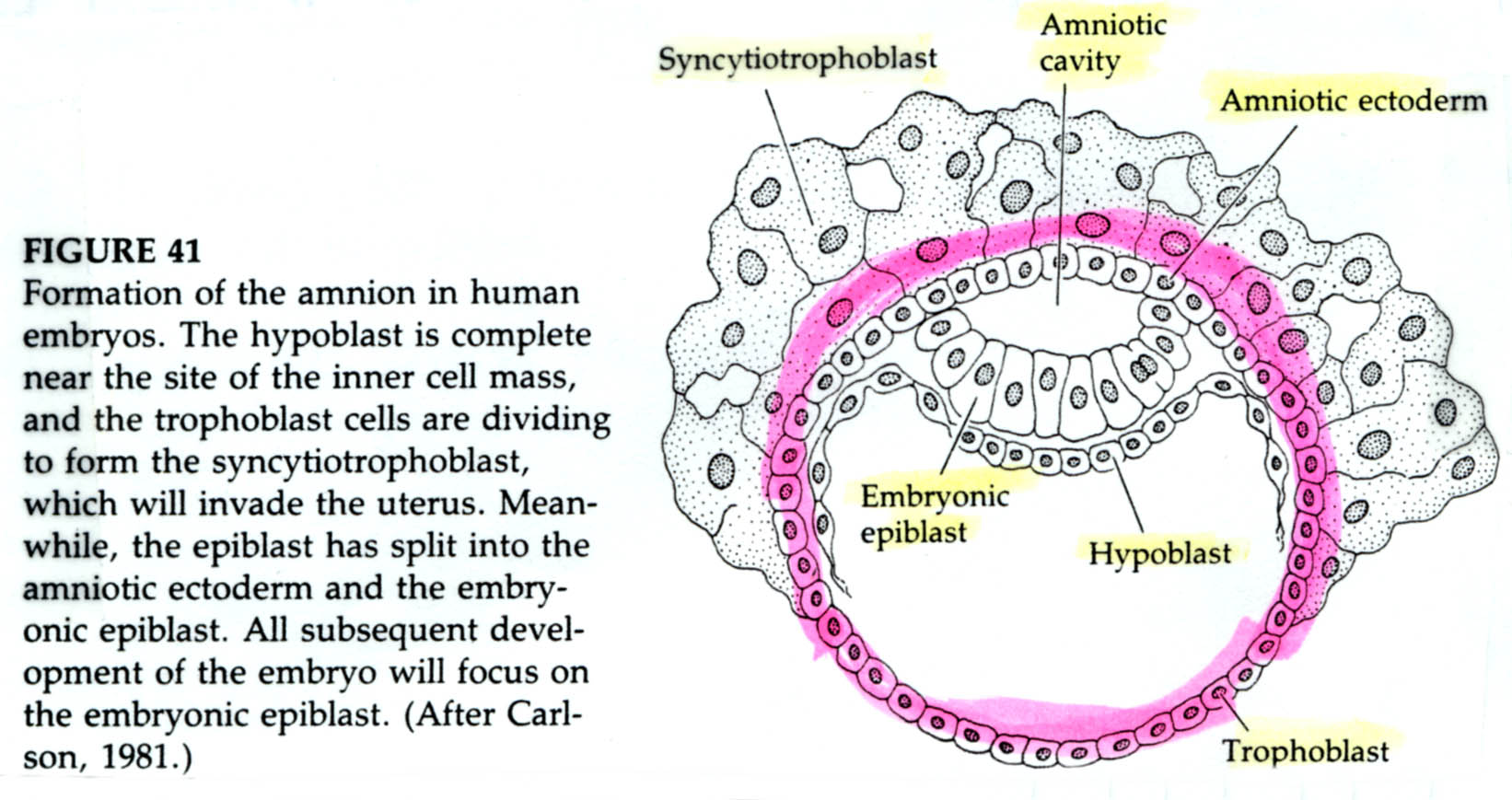


Just as in avian gastrulation cells of the posterior margin migrate inward to form the primitive streak. They migrate anterior to form Hensen’s node. Ingressing cells are coated with HYALURONIC ACID and dramatically change their adhesive interactions with one another and the elelments of the blastocoel. First ingressing cells at Hensen’s node move anterior to form head processes and notochord. Cells ingressing through primitive streak migrate ventrally and latteral to form presumptive mesodermal and endodermal precursors.



While gastrulation to generate the germ layers of the embryo is occuring the extraembryonic cells are beginning the development of the chorian (the embryonic contribution to the placenta). The trophoblastic cells give rise to a layer call the CYTOTROPHOBLAST layer and a population of cells that undergo karyokinesis without cytokinesis. This population of cells consitutes the SYNCYTIOTROPHOBLAST layer. The SYNCYTIOTROPHOBLAST cellsinvades the uterine lining embedding the embryo with the uterus. The uterus sends blood vessels into this area of invading sycytiotrophoblasts. Mesodermal tissue extends outward from the gastrulating embryo forming blood vessels along a connecting stalk that connects the embryo to the trophoblast. This later becomes the UMBILICAL CORD. The trophoblast tissue and mesoderm derive blood vessels constitute the CHORIAN which fused to the uterine wall constitutes the PLACENTA.

The remaining figures show how the organ rudiments develop from the three germ layers. We will discuss this more extensively later in the course when we examine organogenesis.


